Subcutaneous Immunoglobulin (SCIg) infusion – gives patients flexibility and control of their time. Compared to intravenous immunoglobulin (IVIg) infusions, SCIg has fewer side effects and does not need vein access.1-4
| SCIg | IVIg |
|---|---|
| Infuse into fat tissue, not vein | Venous access required |
| Patient self-administered | Healthcare professional required |
| Daily, weekly, biweekly*1 | Administer every 3-4 weeks |
| Gradual absorption (24 – 72 hours)1 | Rapid absorption |
| Fewer peaks and troughs, more consistent Ig levels6 | High peak and low trough levels6 |
| Troughs associated with less infections7 | Troughs had no relation with infection rates7 |
| Pre-medication often not required8 | Pre-medication commonly required |
*Infusion frequency can be tailored to the needs of the individual patients.
Choosing an infusion device is a very important decision. The two main types of infusion pumps are electronic pumps (or constant flow systems) and mechanical pumps (or constant pressure systems).
| Constant Flow Systems | Constant Pressure Systems (KORU Freedom Systems) |
|
|---|---|---|
| Type of infusion | Electronic | Mechanical |
| Software | Programmed to a specific flow rate | No programming |
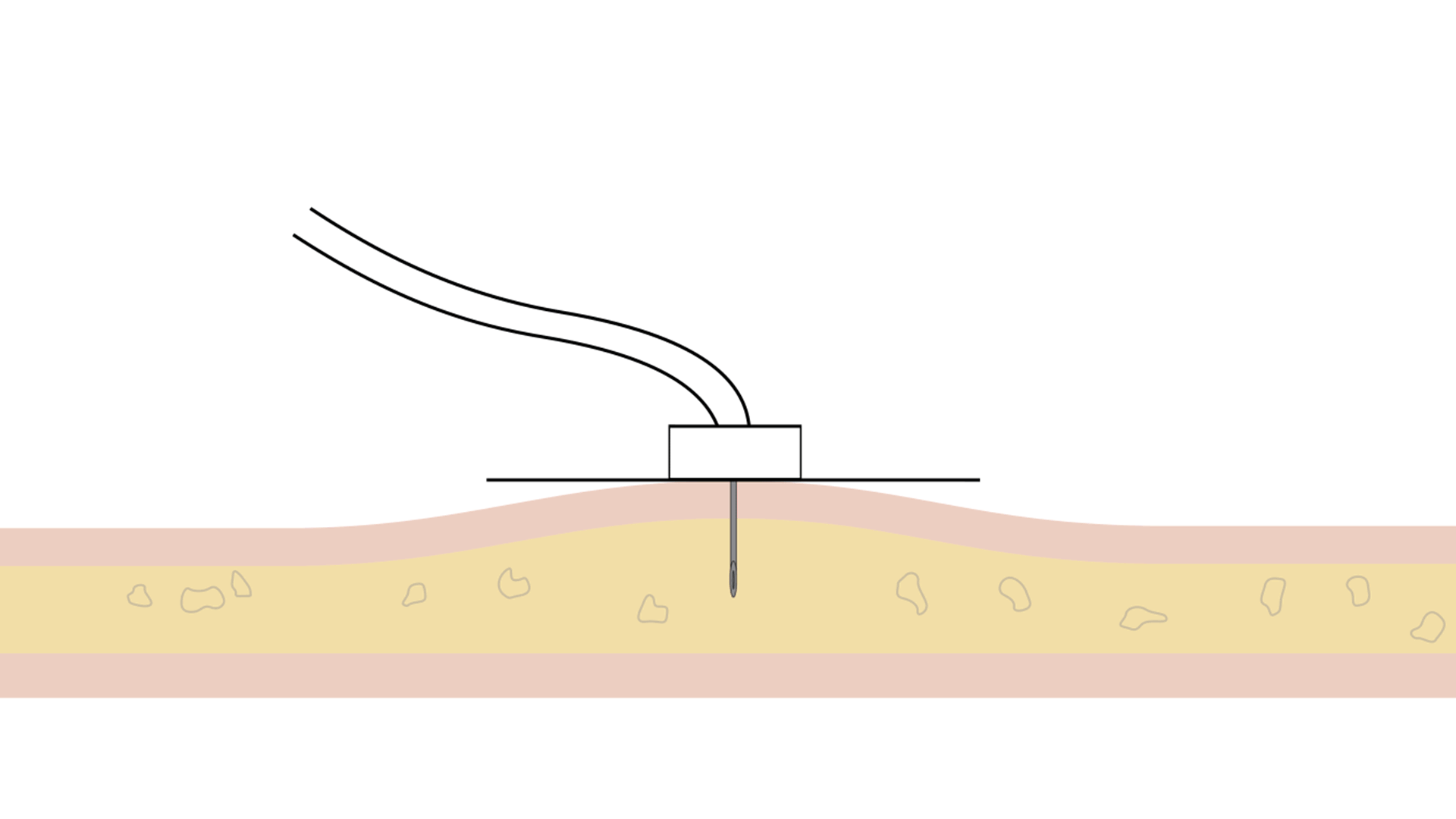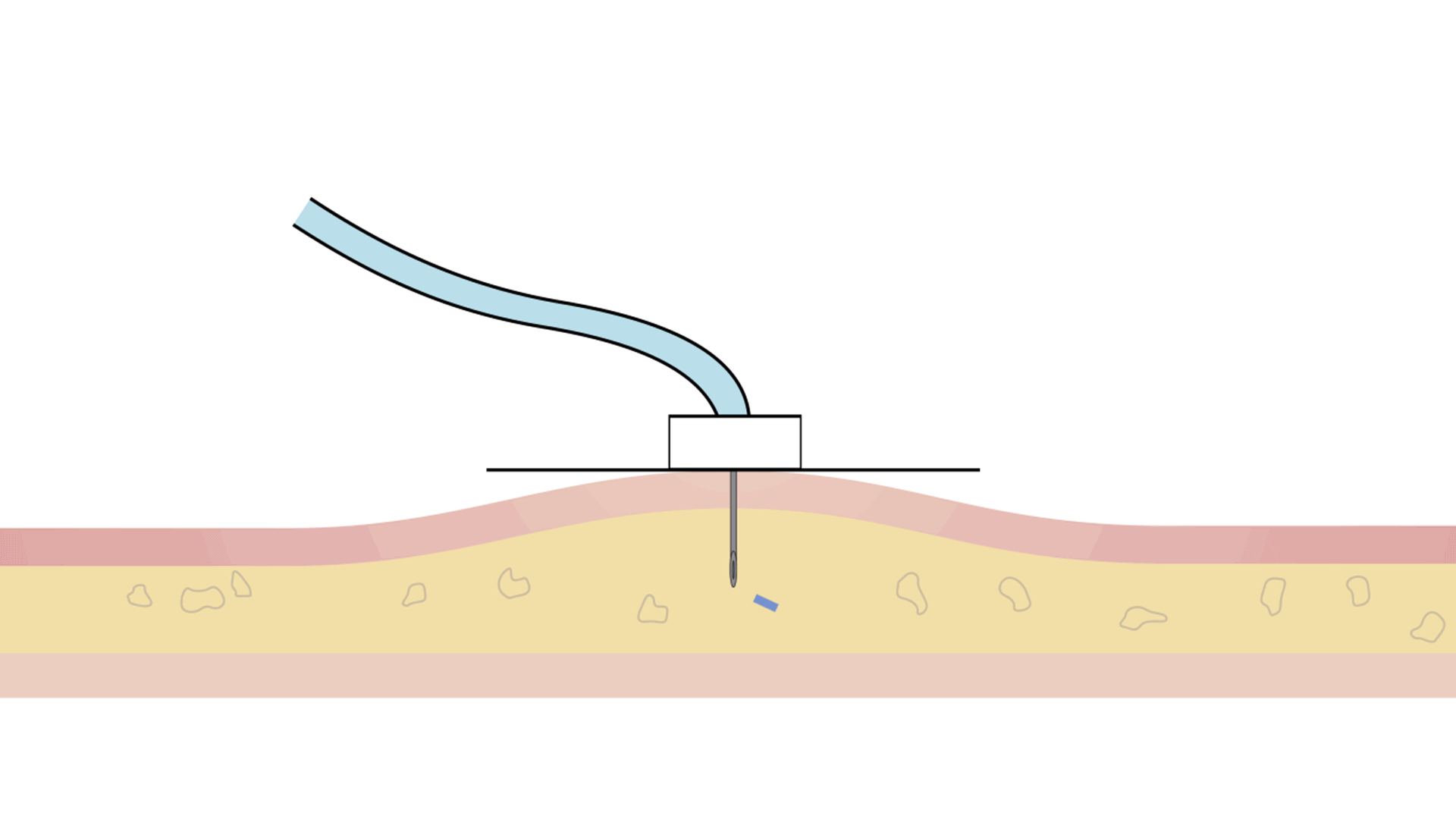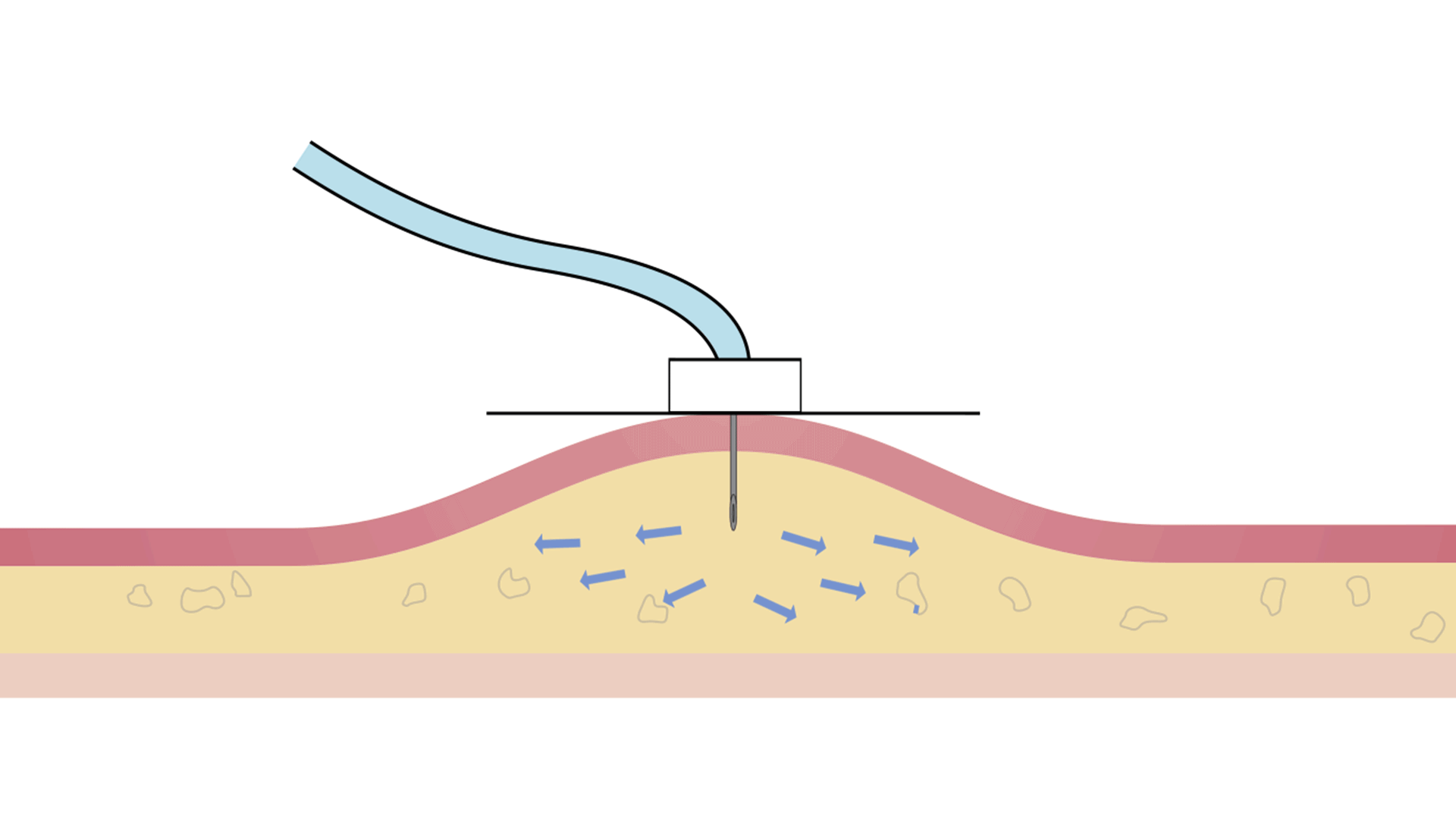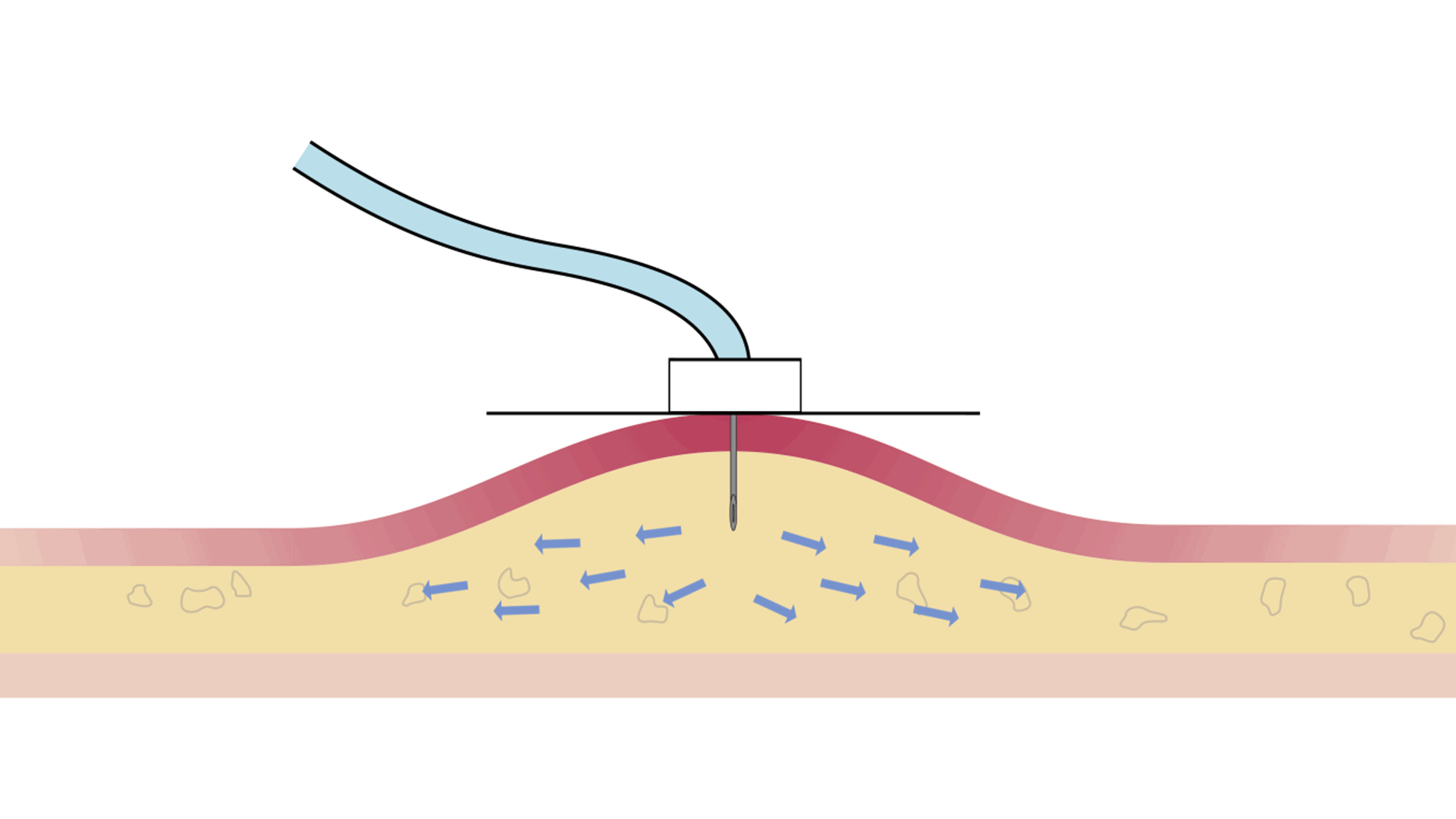
| Response to saturation | By increasing pressure | By slowing down the infusion to maintain the same safe pressure throughout the infusion |
 |  |

Constant Flow Systems

KORU Freedom Systems
The Freedom60® has a simple, ergonomic design that can support large-volume (>30 mL) infusions. The FreedomEDGE® is smaller, portable and suited for small-volume (≤30 mL) infusions.
Freedom60® | FreedomEDGE® |
|
|---|---|---|
 |  |
|
| Infusion Volume | Large volume infusions | Small volume infusions |
| Syringe/Dose drawn from vial | BD® 50mL | BD® 20mL and 30mL |
| Prefilled syringe (PFS) | Hizentra® 50 mL PFS with adapter | Hizentra® 20 mL PFS |

Freedom60®

FreedomEDGE®
The Freedom60® and FreedomEDGE® are specifically indicated for the subcutaneous infusion of the following human plasma-derived immunoglobulins when used according to the FDA approved biologic labeling. TheFreedomEDGE® is additionally indicated for the subcutaneous infusion of Empaveli (pegcetacoplan) when used according to the FDA approved labeling.
Refer to the Freedom60® and FreedomEDGE® Instructions for Use for a full list of cleared indications for use.
Freedom60® | FreedomEDGE® |
|
|---|---|---|
| Cutaquig®, Immune Globulin Subcutaneous (Human) 16.5% Solution (manufactured by Octapharma®) |  | |
| Cuvitru® Immune Globulin Infusion (Human) 20% (manufactured by Takeda®) |  |  |
| Gammagard Liquid®, Immune Globulin Infusion (Human) 10% (manufactured by Takeda®) |  |  |
| Hizentra®, Immune Globulin Subcutaneous (Human) 20% Liquid (manufactured by CSL Behring®) |  |  |
| Hizentra® single-use prefilled syringe |  Hizentra® 50 mL PFS compatible with use of Freedom60® PFS Adapter |  20 mL PFS compatible with FreedomEDGE® Infusion Pump |
| Xembify®, Immune Globulin Subcutaneous (Human) 20% Liquid (manufactured by Grifols®) |  | |
| Empaveli® (pegcetacoplan) injection, 1080 mg/20 ml solution (manufactured by Apellis) |  |
|
| Gammanorm® (Human Normal Immunoglobulin, 165 mg/ml Solution) |  Outside US only |  Outside US only |
| HyQvia® [Immune Globulin Infusion 10% (Human) with Recombinant Human Hyaluronidase] |  Outside US only† |  Outside US only † |
* Only when used with the Freedom60® Prefilled Syringe Adapter. † Only when used with the Precision™ Flow Rate Controller
Freedom60® | FreedomEDGE® |
|
|---|---|---|
| Ertapenem |  |  |
| Meropenem |  |  |
| Oxacillin |  |  |
| Tobramycin |  |  |
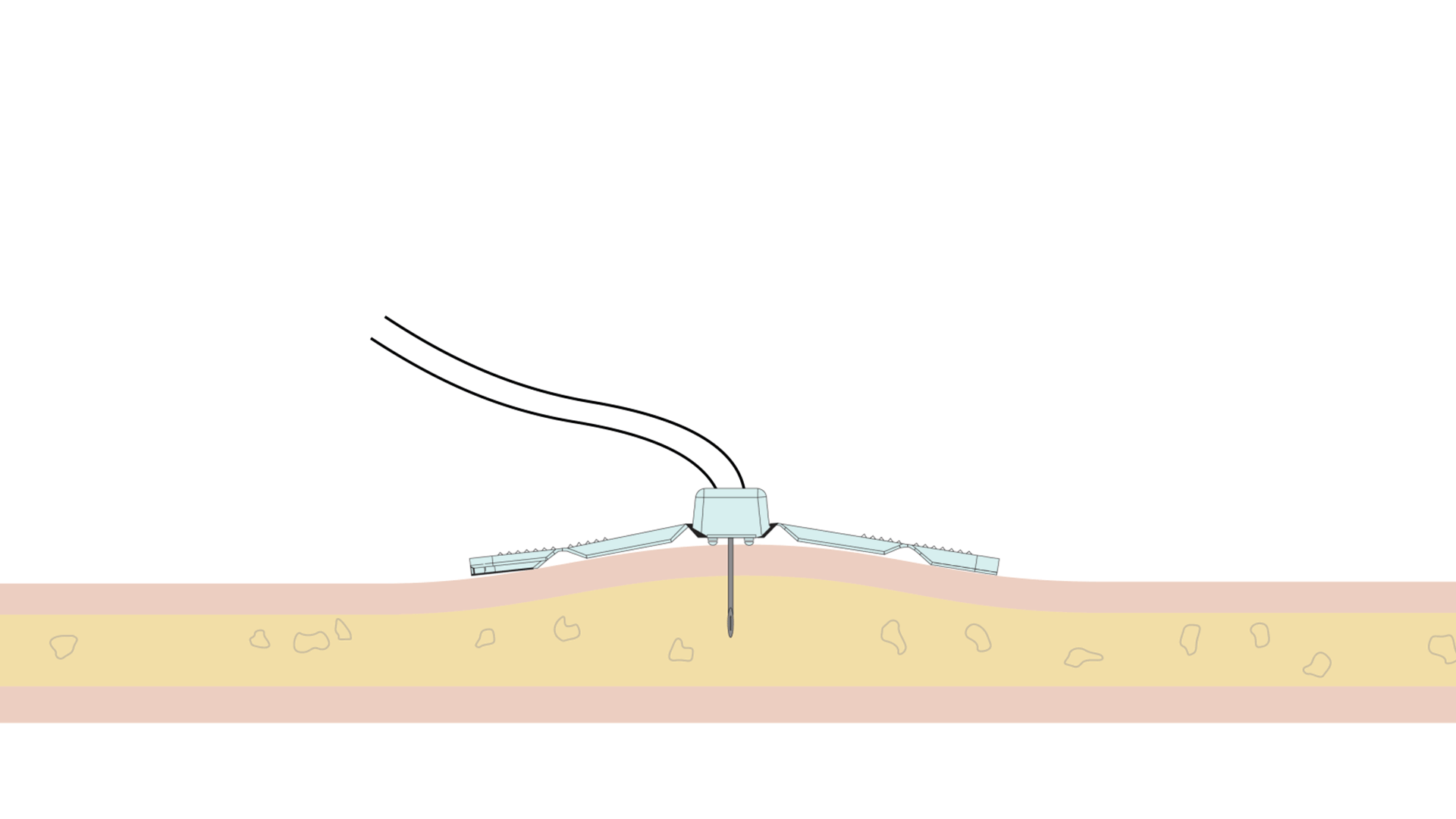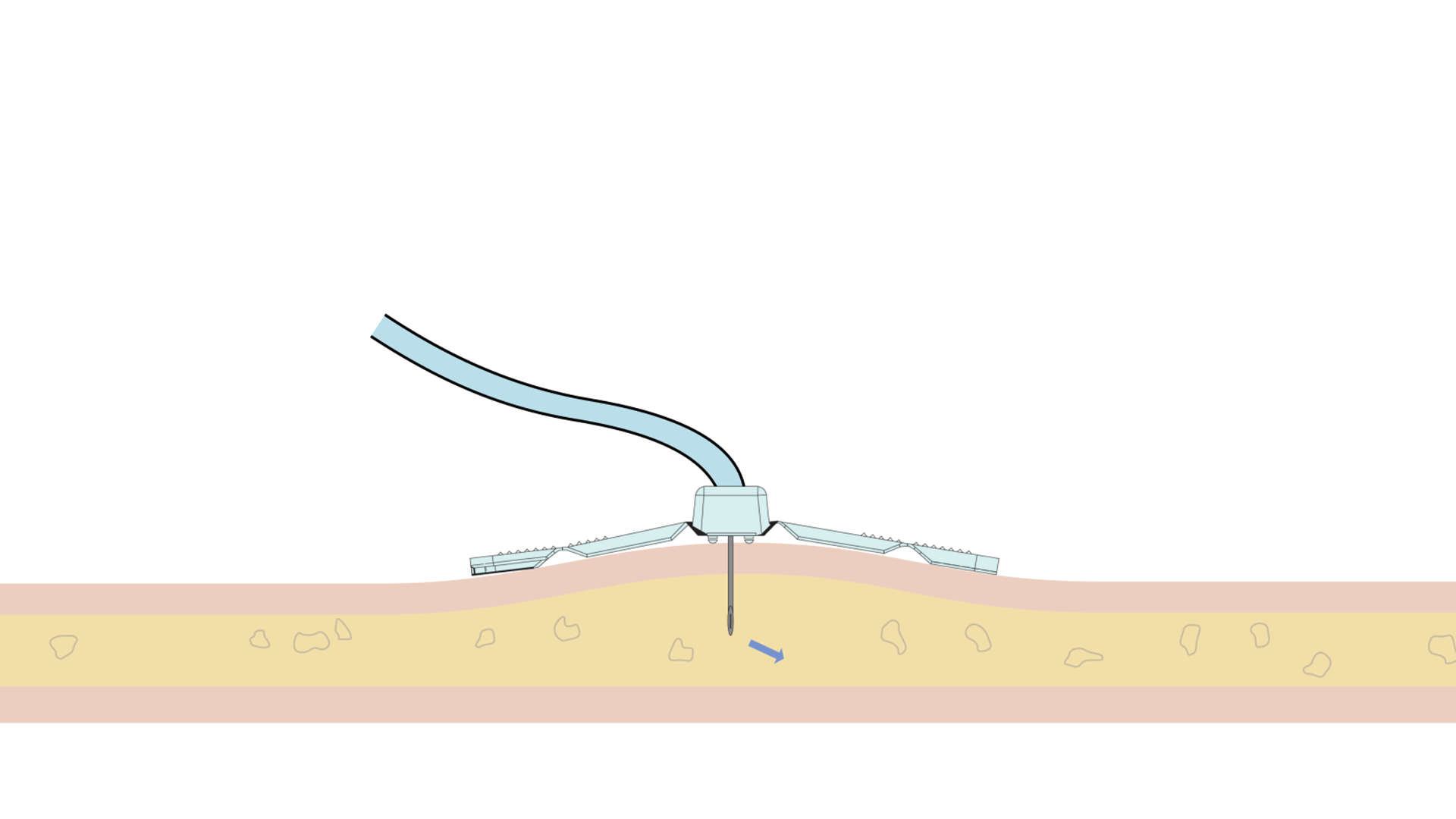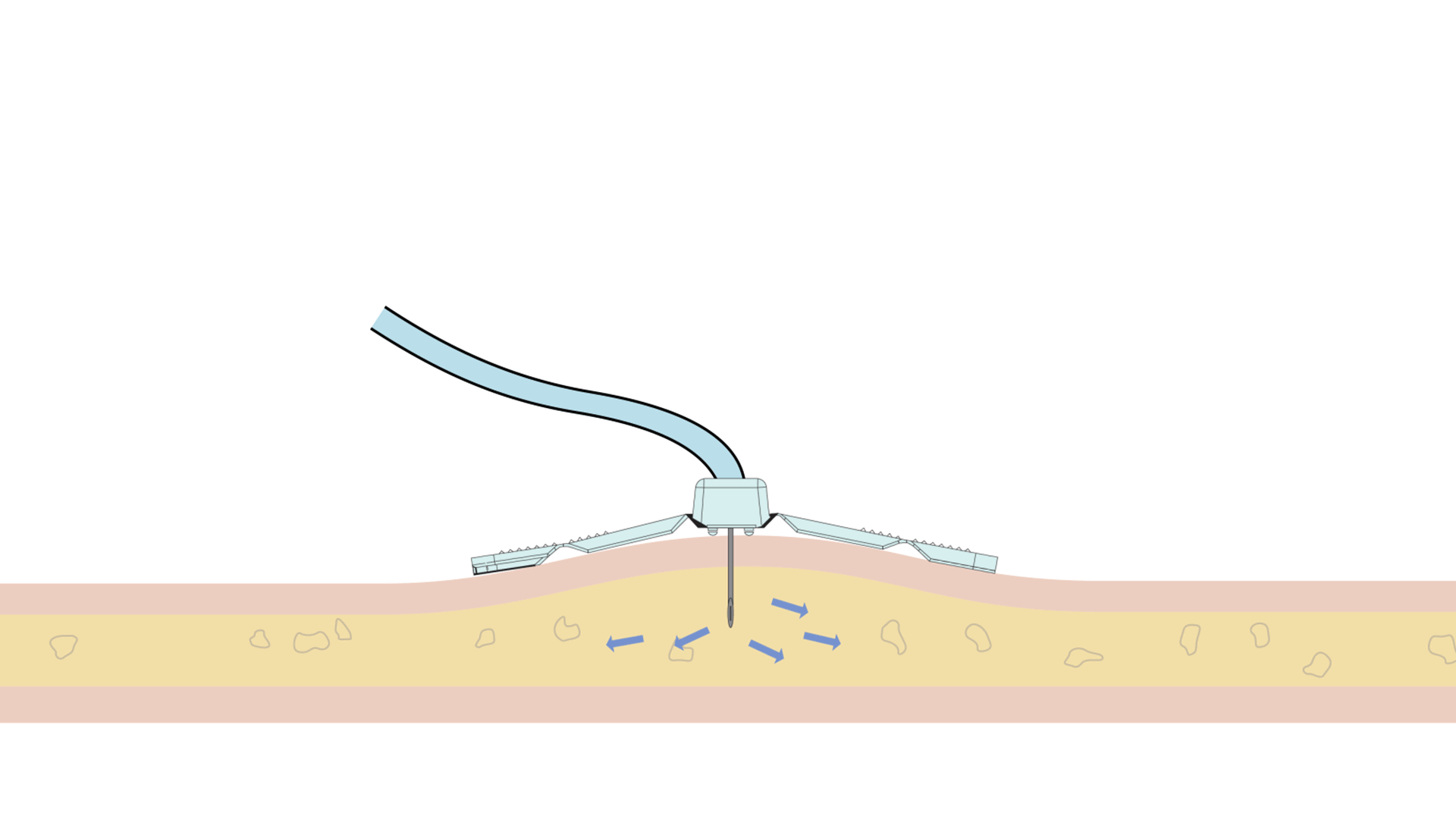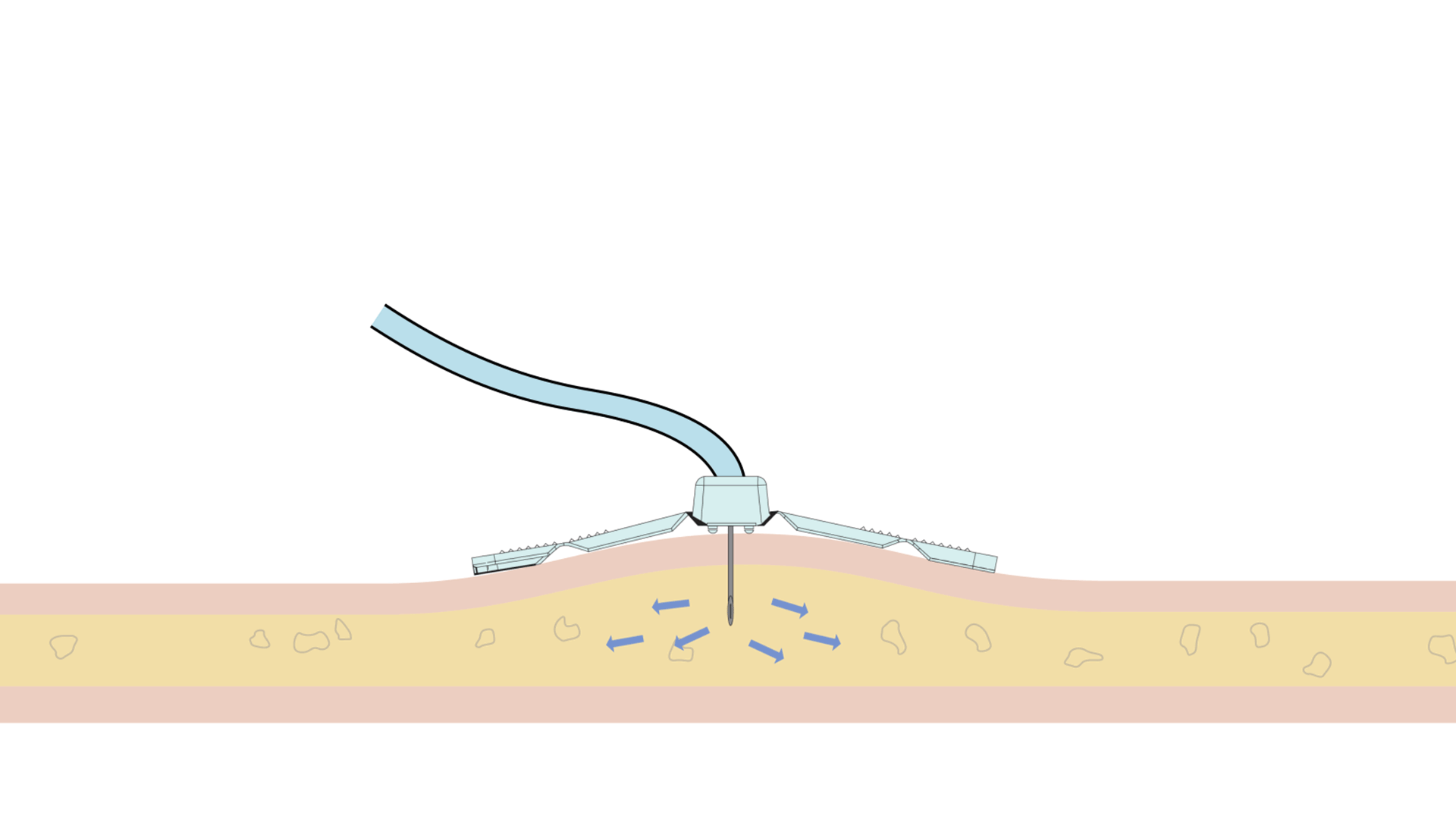
The Freedom™ Infusion System operates at low pressure10 and uses a constant force spring mechanism to apply constant pressure to the syringe. The constant pressure acts as a safety feature of the device and allows the system to automatically decrease the flow rate if there is an increase in resistance at the patient infusion site.10 The system will find a dynamic equilibrium between the increasing resistance and the flow rate. This results in fast and safe infusions with minimal infusion site complications.11
References
Additional Resources
KORU Medical Systems, Inc.
100 Corporate Drive
Mahwah, NJ 07430 USA
Tel: +1-800-624-9600
Fax: +1-845-469-5518
